
Visby Medical
Founded Year
2012Stage
Grant - V | AliveTotal Raised
$470.23MValuation
$0000Last Raised
$3.9M | 3 mos agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-83 points in the past 30 days
About Visby Medical
Visby Medical develops polymerase chain reaction (PCR)-based diagnostic tests for the detection of infectious diseases. The company offers medical solutions for sexual health tests, respiratory health tests, and other infectious diseases. It was formerly known as Click Diagnostics. The company was founded in 2012 and is based in San Jose, California.
Loading...
Loading...
Expert Collections containing Visby Medical
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Visby Medical is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
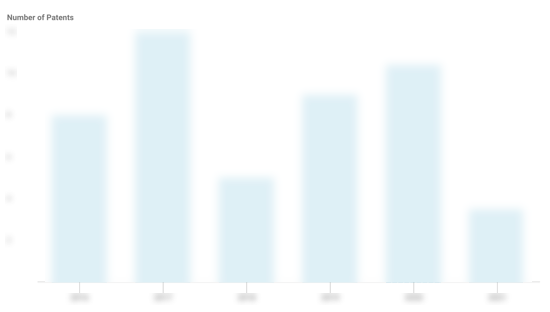
Visby Medical Patents
Visby Medical has filed 39 patents.
The 3 most popular patent topics include:
- molecular biology
- medical tests
- biotechnology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
4/28/2020 | 4/1/2025 | Electronic health records, Health informatics, Blood tests, Medical tests, Sensors | Grant |
Application Date | 4/28/2020 |
|---|---|
Grant Date | 4/1/2025 |
Title | |
Related Topics | Electronic health records, Health informatics, Blood tests, Medical tests, Sensors |
Status | Grant |
Latest Visby Medical News
Apr 7, 2025
Visby Medical has received De Novo authorization from the U.S. Food and Drug Administration (FDA) for its Women’s Sexual Health Test for Over the Counter (OTC) use. According to the company it is the first-ever PCR (polymerase chain reaction) diagnostic device approved by the FDA for OTC home use for any indication. The test allows women to test reliably, rapidly, and privately at home for Chlamydia, Gonorrhea, and Trichomoniasis – the three most common curable sexually transmitted infections (STIs). Unlike other home-based STI tests on the market that require mailing samples to a laboratory, Visby’s technology provides results directly to users within 30 minutes using PCR technology, widely recognized as the gold standard in diagnostic accuracy. “This approval is not just a milestone for Visby Medical but marks a transformative moment in medical diagnostics,” said Adam de la Zerda, PhD, Founder and CEO of Visby Medical. “We’ve achieved something incredible; our palm-sized, single-use PCR test is simple to use and replaces a bulky, large, expensive laboratory instrument. After 12 years of development, our device delivers rapid, reliable results directly into the hands of consumers, with unparalleled convenience and privacy. We also built a state-of-the-art, fully automated manufacturing line ready to rapidly scale production in anticipation of growing consumer demand.” The technology has undergone extensive clinical validation, with studies involving over 2,000 lay users demonstrating that the Visby Medical Women’s Sexual Health Test delivers accuracy comparable to traditional laboratory-based PCR machines. This level of accuracy enables healthcare providers to confidently prescribe treatment based on test results. The system includes an intuitive companion app that guides users through the entire testing process—from sample collection to test execution and result interpretation—while providing a seamless connection to further care options. “The clinical significance of bringing a rapid, highly accurate PCR diagnostic test into the home environment cannot be overstated,” said Gary Schoolnik, MD, Chief Medical Officer of Visby Medical. “Extensive clinical studies validate that this test empowers women to quickly understand what steps to take next, giving them the privacy, control, and confidence to seek the care they need. Importantly, many patients infected with these STIs are non-symptomatic, yet they can still suffer serious long-term health consequences. Our test directly addresses this silent epidemic by enabling detection and treatment.” You May Also Like
Visby Medical Frequently Asked Questions (FAQ)
When was Visby Medical founded?
Visby Medical was founded in 2012.
Where is Visby Medical's headquarters?
Visby Medical's headquarters is located at 3010 North First Street, San Jose.
What is Visby Medical's latest funding round?
Visby Medical's latest funding round is Grant - V.
How much did Visby Medical raise?
Visby Medical raised a total of $470.23M.
Who are the investors of Visby Medical?
Investors of Visby Medical include CARB-X, Pitango Venture Capital, John Doerr, Artiman Ventures, Blue Water Life Science Advisors and 22 more.
Who are Visby Medical's competitors?
Competitors of Visby Medical include Sense Biodetection and 1 more.
Loading...
Compare Visby Medical to Competitors
Nostics works as a healthcare technology company that specializes in point-of-care diagnostics within the medical diagnostics industry. The company provides pathogen identification solutions that assist healthcare providers in prescribing antibiotics during the initial patient consultation. Nostics' technology utilizes nanotechnology, photonics, and machine learning, producing results. Nostics was formerly known as Spektrax. It was founded in 2020 and is based in Amsterdam, Netherlands.

Biomerieux specializes in in vitro diagnostics within the healthcare sector, focusing on the development and manufacturing of diagnostic tests. The company offers a broad range of products that enable rapid and accurate detection of infectious diseases, antimicrobial resistance, and food safety concerns, as well as solutions for pharmaceutical quality control. These products serve various sectors including healthcare, food industry, and pharmaceuticals. It was founded in 1963 and is based in Marcy L'Etoile, France.

Oxford Nanopore Technologies (LSE: ONT.L) specializes in deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) sequencing technology within the biotechnology sector. The company offers products and solutions for DNA and RNA sequencing, including library preparation, sequencing devices, flow cells, and data analysis tools. Its products enable the analysis of genetic material for research in areas such as microbiology, human genetics, cancer, and environmental studies. It was founded in 2005 and is based in Oxford, United Kingdom.

Cue Health serves as a healthcare technology company specializing in diagnostic testing within the health sector. The company offers a portable molecular testing platform that provides lab-quality, actionable health insights quickly and conveniently. Cue Health primarily serves the healthcare industry, including clinics and individuals seeking at-home diagnostic solutions. It was founded in 2010 and is based in San Diego, California. In May 2024, Cue Health filed for bankruptcy.

Lucira Health specializes in at-home molecular testing for the healthcare sector. The company offers a test that identifies whether viral respiratory symptoms are due to COVID-19 or the flu, with results available in approximately 30 minutes. This test is for use at home and is available over-the-counter. Lucira Health was formerly known as Diassess. It was founded in 2013 and is based in Emeryville, California. In February 2023, Lucira Health filed for bankruptcy.

Sense Biodetection operates as a molecular diagnostics company. It designs and develops proprietary molecular and device technologies. The company serves hospitals, urgent care, nursing homes, workplace and campus health, community health, and travel sectors. The company was founded in 2014 and is based in Abingdon, U.K. In February 2023, Sense Biodetection was acquired by Sherlock Biosciences. The terms of the transaction were not disclosed.
Loading...
