From deal activity to top investors, we analyze the shifting landscape for AI in clinical trials to determine how the tech is transforming drug development for the better.
This is the third and final report in a 3-part series on how AI is reshaping discovery, preclinical, and clinical research in drug R&D. Read part 1 on the discovery phase and part 2 on preclinical development.
Clinical development represents one of pharma’s costliest and riskiest investments.
Trials average $55M each, per a study in JAMA Health Forum, and can take more than a decade to complete. However, over 90% of drugs still fail. AI is emerging as a game-changing force in clinical development, tackling its biggest pain points head-on.
For example, AI-driven patient recruitment solutions automate key administrative processes to help speed up trial enrollment, while the integration of AI into data analysis tools enables researchers to analyze much larger and more complex datasets than ever before.
Key takeaways from our analysis of the clinical development AI space include:
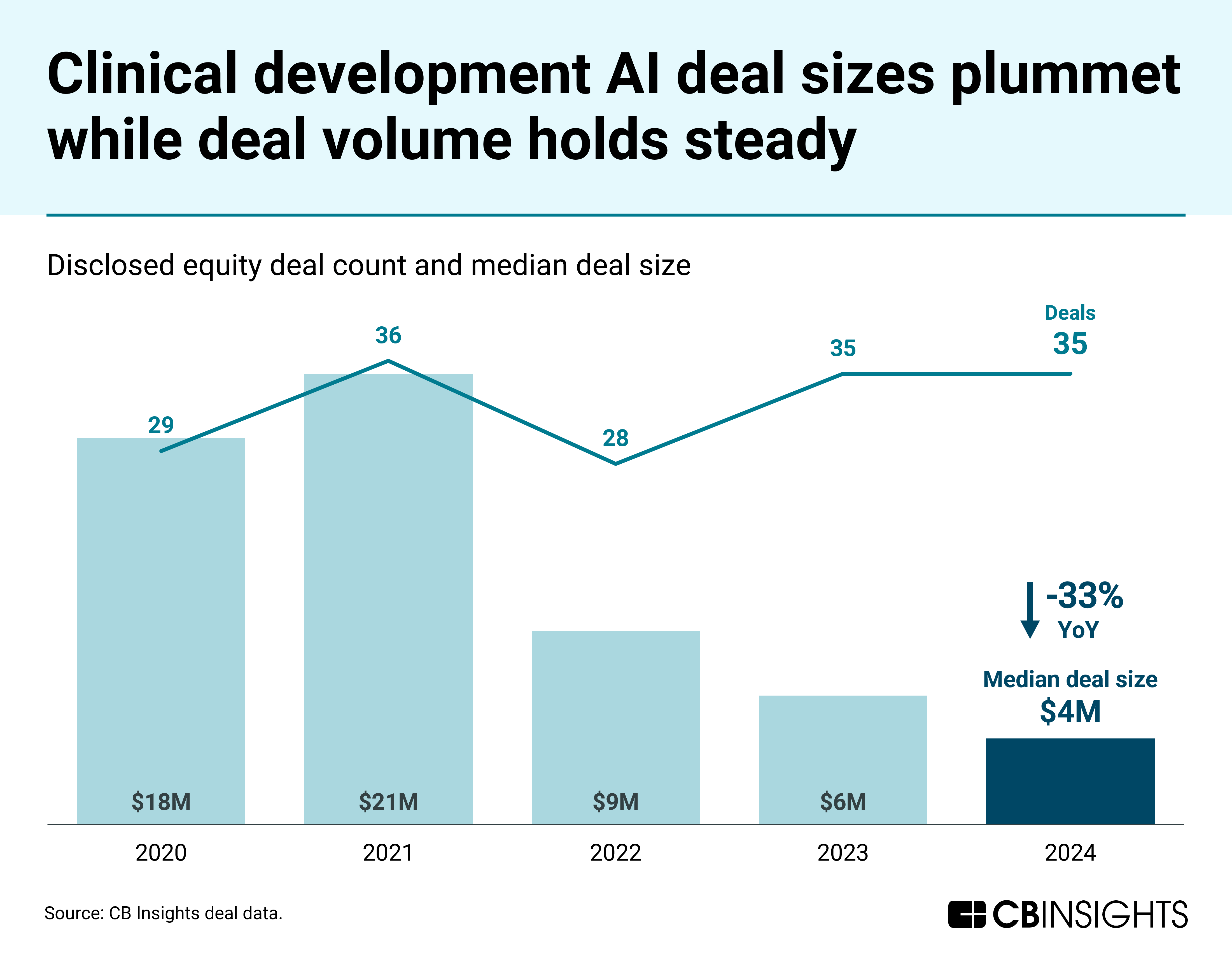
- Clinical development AI maintains strong operational momentum despite reduced capital availability. While median deal size in the space has fallen each year since 2021, the sector continues to expand through stable deal volume, headcount growth, and a rising rate of new company formations.
- Patient recruitment and data analysis are the most active clinical development AI markets. Since 2023, these markets have led in clinical development AI funding and deal share, collectively capturing 73% of equity funding and 59% of deals. Solutions in both spaces are drawing attention for their ability to address critical bottlenecks in clinical trials, focusing on accelerating patient enrollment and large-scale data analysis.
- Alphabet has established a dominant competitive position in clinical development AI. Since 2020, it has backed 6 equity rounds to the space, exceeding the deal volume from any single big pharma or big tech company. Beyond equity investments, Alphabet also supports clinical development AI companies through its accelerator programs and Google Cloud infrastructure collaborations, creating a sphere of influence that can be monitored to help identify future market leaders.
Top applications for AI in the clinical development phase
Clinical development begins with the onset of human trials and culminates, for US programs, in the filing of a New Drug Application (NDA) or Biologics License Application (BLA) with the Food and Drug Administration.
AI promises to improve 8 different facets of clinical development, each presenting distinct challenges in bringing a new drug to market. We explore these areas below and then highlight companies that have recently demonstrated market traction.
Want to see more research? Join a demo of the CB Insights platform.
If you’re already a customer, log in here.