AI promises to accelerate the path from bench to bedside. Using CB Insights data, we highlight emerging markets in the preclinical phase that are ripe for pharma investments and partnerships.
This is the second report in a 3-part series on how AI is reshaping discovery, preclinical, and clinical research in drug R&D. Read part 1 on the discovery phase here.
For pharma companies, it’s critical to maximize efficiency in preclinical development — the stage after drug discovery and before clinical trials — as poor processes can mask early warning signs of drug candidates that are likely to fail.
The stakes are high: Studies show that 90% of drugs fail in clinical trials. In Phase 1 trials alone — which have a median cost of $3.4M per trial — 40% of drug candidates fail.
Just as in the discovery phase, AI holds significant potential to enhance the speed and accuracy of preclinical development — thereby reducing trial costs and increasing the likelihood of success.
Our analysis reveals:
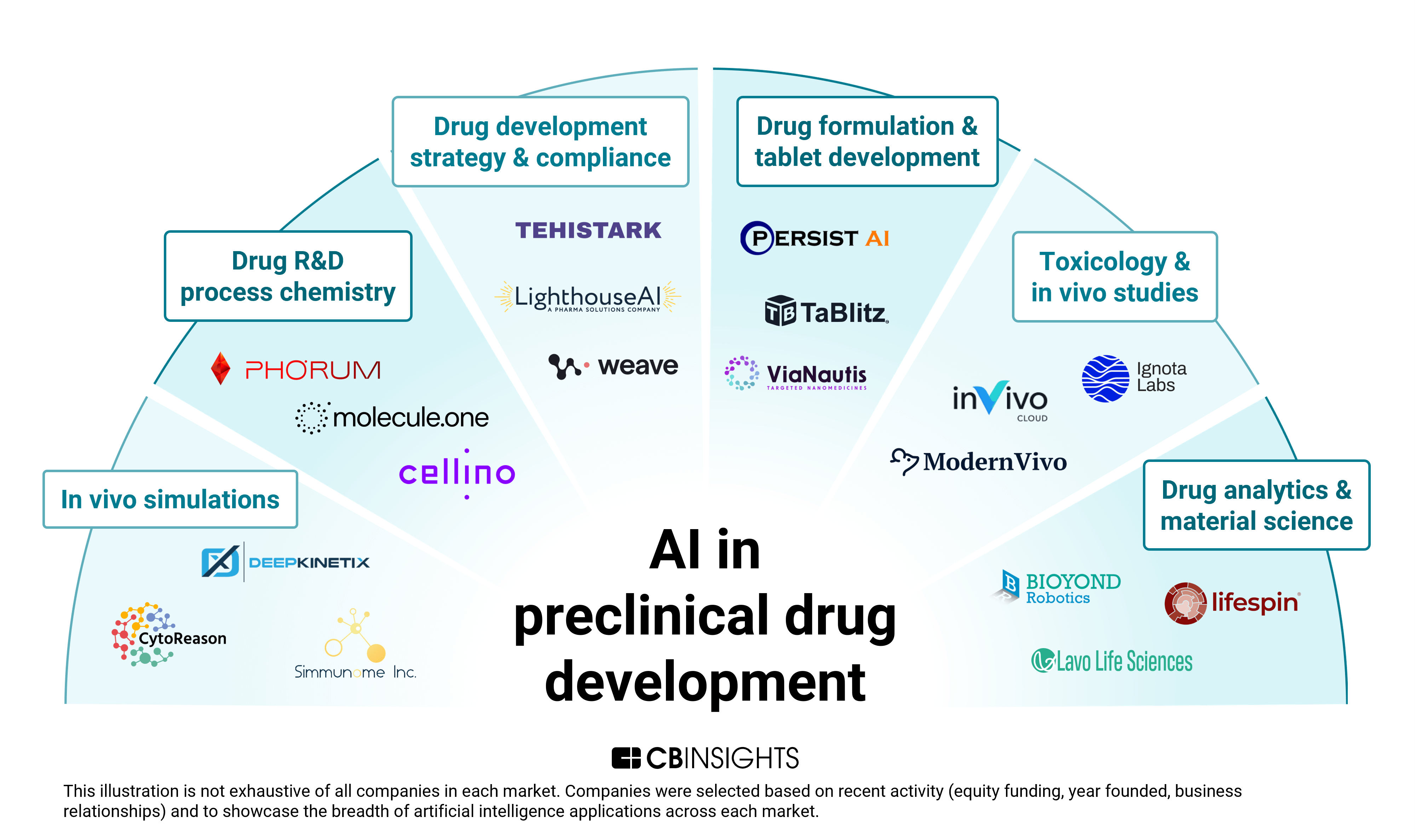
- The preclinical AI landscape is highly nascent. The space has one-fifth as many startups as drug discovery AI, with half founded since 2020. It has also seen far less mid- to late-stage activity, having secured just 9 Series B or later rounds (vs. 176 for drug discovery AI). This relatively untapped, young market presents opportunities for pharma companies and contract development and manufacturing organizations (CDMOs) to get involved early via acquisitions and partnerships. Unlike discovery, preclinical AI solutions are niche and mid-pipeline, and they depend on pharma collaboration for validation.
- AI models take on the human drug response. Among the preclinical AI companies we’ve identified, over 25% focus on biosimulations to forecast clinical outcomes. Compared to traditional modeling techniques, the use of AI is powering more sophisticated simulations that predict drug properties and uncover patterns in complex biological data. For pharmaceutical companies, this AI-powered approach promises to both accelerate drug development and reduce costly trial failures.
- Formulation development emerges as AI’s new frontier. Since 2023, more than half of preclinical AI investment dollars have gone to formulation and tablet development. The concentration of funding targets an industry pain point: optimizing delivery systems for complex biologics and poorly soluble drugs. Investors are betting on AI to push the frontier of drug delivery optimization and slash development times.
Source: CB Insights — AI-driven preclinical development companies
Top applications for AI in the preclinical development phase
Preclinical development serves as the bridge between early-stage research and clinical trials that, for US programs, culminates in an Investigational New Drug (IND) application. This preclinical phase involves testing drug candidates through in vitro, in vivo, and in silico studies to establish safety and efficacy before human trials begin.
Want to see more research? Join a demo of the CB Insights platform.
If you’re already a customer, log in here.