
IntraBio
Founded Year
2015Stage
Unattributed - II | AliveTotal Raised
$50.57MValuation
$0000Last Raised
$40.27M | 1 yr agoAbout IntraBio
IntraBio operates as a biopharmaceutical company focused on the research, development, and commercialization of therapies for rare and neurodegenerative diseases. The company's main offerings include clinical trials and development programs for treatments that address conditions with high unmet medical needs. IntraBio primarily serves the healthcare sector, particularly in areas related to neurodegeneration and rare disease treatment. It was founded in 2015 and is based in Austin, Texas.
Loading...
Loading...
Expert Collections containing IntraBio
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
IntraBio is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
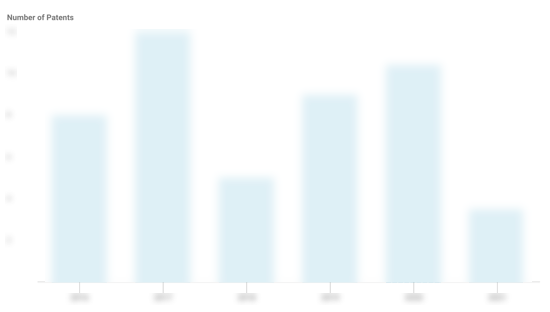
IntraBio Patents
IntraBio has filed 22 patents.
The 3 most popular patent topics include:
- rare diseases
- autosomal recessive disorders
- neurological disorders
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
8/11/2017 | 11/19/2024 | Rare diseases, Neurological disorders, Autosomal recessive disorders, Neuroscience, Neurology | Grant |
Application Date | 8/11/2017 |
|---|---|
Grant Date | 11/19/2024 |
Title | |
Related Topics | Rare diseases, Neurological disorders, Autosomal recessive disorders, Neuroscience, Neurology |
Status | Grant |
Latest IntraBio News
Mar 17, 2025
Research and Markets Dublin, March 17, 2025 (GLOBE NEWSWIRE) -- The "Tay Sachs Disease Pipeline Insight Analysis Report" drug pipelines has been added to ResearchAndMarkets.com's offering. The Tay-Sachs treatment pipeline analysis provides an overview of recent advancements and ongoing clinical trials. The report highlights progress in developing novel therapies, including enzyme replacement therapies, substrate reduction therapies, and gene therapies, aiming for effective disease control and long-term management. It covers innovative approaches such as personalized medicine, which tailors treatments based on individual patient profiles, and advanced diagnostic technologies for improved treatment efficacy. Tay Sachs Drug Pipeline Outlook Tay-Sachs disease is a fatal genetic disorder resulting from a deficiency in the enzyme hexosaminidase A. This deficiency leads to the accumulation of GM2 ganglioside in neurons, causing progressive neurological damage. Symptoms include developmental delays, muscle weakness, vision and hearing loss, and severe neurological impairment. Recent advancements focus on enzyme replacement and gene therapies to address the underlying genetic cause and improve patient outcomes. Recent advancements focus on targeted therapies and personalized medicine to improve patient outcomes by addressing the specific molecular and genetic characteristics of the disease. In 2023, significant advancements in T-cell lymphoma treatment have been made. The FDA approved IntraBio's Investigational New Drug (IND) application for their lead compound, IB1001. This approval allows IntraBio to commence clinical trials for Tay-Sachs and Sandhoff diseases in the United States and Europe. IB1001, an oral acetylleucine powder, is designed to treat these lysosomal storage disorders by potentially restoring the deficient enzyme activities that cause these conditions. Key Takeaways Key players in the Tay-Sachs drug pipeline market include Genzyme, a Sanofi Company, and Aldagen among others. These companies are at the forefront of developing cutting-edge therapies to improve patient outcomes. The drug pipeline for Tay-Sachs includes promising candidates such as venglustat (GZ402671) and IB1001. These treatments focus on reducing the accumulation of GM2 ganglioside, enhancing lysosomal function, and providing innovative approaches to manage symptoms and improve survival rates. Regulatory agencies are encouraging the development of novel Tay-Sachs treatments by providing incentives such as fast-track designations and priority reviews. This support is crucial for facilitating quicker access to new and effective therapies for patients, addressing the urgent need for better treatment options in this challenging condition. Tay Sachs - Pipeline Drug Profiles Venglustat (GZ402671): Venglustat is an investigational substrate reduction therapy aimed at reducing the accumulation of GM2 ganglioside. Developed by Genzyme, it targets the underlying cause of Tay-Sachs by inhibiting glucosylceramide synthase. IB1001: IB1001 is a gene therapy candidate designed to deliver a functional HEXA gene to patients, thereby restoring hexosaminidase A enzyme activity and preventing GM2 ganglioside buildup. Companies Featured
IntraBio Frequently Asked Questions (FAQ)
When was IntraBio founded?
IntraBio was founded in 2015.
Where is IntraBio's headquarters?
IntraBio's headquarters is located at 201 West 5th Street, Austin.
What is IntraBio's latest funding round?
IntraBio's latest funding round is Unattributed - II.
How much did IntraBio raise?
IntraBio raised a total of $50.57M.
Loading...
Loading...
