
Kallyope
Founded Year
2015Stage
Grant | AliveTotal Raised
$487.2MLast Raised
$8.2M | 2 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-117 points in the past 30 days
About Kallyope
Kallyope is a biotechnology company focused on developing oral, small-molecule therapeutics for metabolic, neurological, and gastrointestinal diseases. The company leverages its Klarity™ platform to discover medicines that address conditions such as obesity, diabetes, migraines, and celiac disease through the exploration of the gut-brain axis. Kallyope's innovative approach combines a broad set of integrated technologies to enhance the understanding of gut biology and interorgan signaling pathways. It was founded in 2015 and is based in New York, New York.
Loading...
Loading...
Expert Collections containing Kallyope
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Kallyope is included in 2 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
Game Changers 2018
36 items
Our selected startups are high-momentum companies pioneering technology with the potential to transform society and economies for the better.
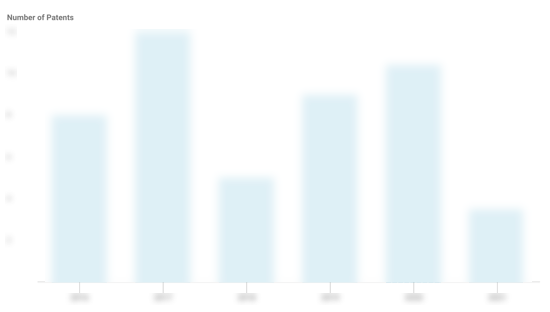
Kallyope Patents
Kallyope has filed 18 patents.
The 3 most popular patent topics include:
- autoimmune diseases
- gastrointestinal tract disorders
- inflammations
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
5/16/2022 | 4/1/2025 | Gastrointestinal tract disorders, Inflammations, Autoimmune diseases, Rare diseases, Diseases of intestines | Grant |
Application Date | 5/16/2022 |
|---|---|
Grant Date | 4/1/2025 |
Title | |
Related Topics | Gastrointestinal tract disorders, Inflammations, Autoimmune diseases, Rare diseases, Diseases of intestines |
Status | Grant |
Latest Kallyope News
Mar 5, 2025
Newsletter Strategist Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox. Good morning! Today, we see a mixed reaction among conservative-leaning scientists following the Trump administration’s swift changes in federal health and science agencies, and we hear one idea on how Canada could respond to the new 25% tariffs. advertisement The need-to-know this morning Danish drugmaker Orbis Medicines, a privately held developer of oral macrocycle medicines, named Mikael Dolsten as its new board chairman. Dolsten was formerly the chief scientific officer at Pfizer. Jazz Pharmaceuticals said it will acquire Chimerix, maker of a treatment for a rare brain tumor, for $935 million. The deal values Chimerix at $8.55 per share, or a 72% premium to its Tuesday closing price. The Chimerix drug, called dordaviprone, is being reviewed by the FDA to treat patients with H3 K27M-mutant diffuse glioma, a rare, high-grade brain tumor that most commonly affects children and young adults. How the race for the next obesity drug is shaping up From STAT’s Allison DeAngelis: The drug industry’s love affair with obesity drugs has continued into 2025, though some companies have experienced heartbreak. BioAge, a startup focused on aging-related conditions, scrapped its lead drug azelaprag in January. The company had shut down a Phase 2 trial of the drug after spotting elevated liver enzymes in participants’ blood samples. The drug targeted amylin receptors, which some drug developers believe could cause fewer side effects and preserve lean mass. BioAge plans to advance another amylin-targeting obesity treatment by the end of the year. Kallyope also reported disappointing results from a Phase 2 trial testing two of its oral drugs: K-757 and K-833. On its own, K-757 didn’t induce a statistically significant amount of weight loss. The drug fared better when paired with K-833, leading to roughly 3% weight loss after 13 weeks of treatment — statistically significant, but well below the type of weight loss tirzepetide and semaglutide induce. advertisement These updates and more are reflected in the latest update of STAT’s Obesity Drug Tracker , which gives a comprehensive look at the status and approaches that dozens of drug companies are taking to help people lose weight. While some drugs have fallen off, more have been added: AbbVie recently jumped into the obesity craze by licensing an experimental medication from Danish company Gubra . You can check out what other drugs Gubra has in the works on STAT’s tracker. Bobby Jindal on ending global price disparities President Trump has vowed to end the chronic disease epidemic, and to do that at home, he might start by looking abroad, former Louisiana governor Bobby Jindal and Charlie Katebi, deputy director of the Center for a Healthy America at the America First Policy Institute, write in a new First Opinion. The pair argue that other wealthy countries refuse to pay fair market prices for pharmaceuticals, leaving Americans to bear the brunt of drug development costs. This freeloading, they write, “allows other countries to enjoy the benefits of American drug innovations, without paying the necessary costs to develop them.” Among their proposed solutions: The Trump administration could leverage policies such as the International Price Index and Most Favored Nation pilot programs to encourage other countries’ investment in greater drug development. How Canada could use drug patents to respond to Trump’s tariffs The Trump administration is levying 25% tariffs on goods from Canada. One way for Canada to respond, McGill University law and medicine professor Richard Gold tells STAT’s Ed Silverman, might be to suspend U.S. pharmaceutical patents to pressure drugmakers into lobbying against the trade measures. Under Canada’s Patent Act, the government could issue compulsory licenses, allowing generic companies to produce U.S.-patented medicines early. This strategy could accelerate the entry of generic drugs into the market — and force American companies to push Trump to uphold trade agreements. “This would tell the Americans that you don’t get the benefit of a free trade agreement if you don’t honor it and it will hurt you, not with a tariff but by taking away things you bargained for, since you’re not willing to pay,” Gold said. “That’s the message.” advertisement A mixed review from scientists who embraced Trump Plenty of scientists cheered the Trump administration’s plans to shake up the country’s health and science milieu. Take Leslie Bienan, a zoonotic disease researcher at Oregon Health & Science University, who was thrilled her old friend Jay Bhattacharya had been tapped to head the NIH. Now, she’s not so enthused. “What’s happening now just seems like generalized chaos,” she told STAT’s Jason Mast. “I certainly don’t know, and I don’t know anybody does, what things will look like in six months. I hope not like this!” Jason spoke with two dozen people who had argued for institutional reform or expressed openness to the new administration — and found decidedly mixed emotions about the first six weeks.
Kallyope Frequently Asked Questions (FAQ)
When was Kallyope founded?
Kallyope was founded in 2015.
Where is Kallyope's headquarters?
Kallyope's headquarters is located at 430 East 29th Street, New York.
What is Kallyope's latest funding round?
Kallyope's latest funding round is Grant.
How much did Kallyope raise?
Kallyope raised a total of $487.2M.
Who are the investors of Kallyope?
Investors of Kallyope include Bill & Melinda Gates Foundation, The Column Group, Alexandria Venture Investments, Polaris Partners, Lux Capital and 15 more.
Loading...
Loading...
