Medable
Founded Year
2012Stage
Series D | AliveTotal Raised
$533.75MValuation
$0000Last Raised
$304M | 3 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-16 points in the past 30 days
About Medable
Medable specializes in providing digital clinical trial software solutions within the healthcare and pharmaceutical sectors. The company offers a comprehensive platform that facilitates the management of clinical trials, including tools for remote data collection, electronic consent (eConsent), patient-reported outcomes (ePRO), and clinical outcome assessments (eCOA), all designed to streamline the trial process and enhance data quality. Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.
Loading...
ESPs containing Medable
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The decentralized clinical trials platforms market specifically encompasses software and technology solutions designed to facilitate and manage remote clinical trials. These platforms integrate various components such as telemedicine, mobile health applications, and wearable device data collection to enable trial activities without frequent site visits. By streamlining remote participation, these …
Medable named as Leader among 15 other companies, including Medidata, ObvioHealth, and Huma.
Medable's Products & Differentiators
Medable Platform
To create a seamless experience for site and patients, all of our products are integrated into a single platform. The core Platform contains portals for the site, patient, and sponsor in both web and mobile modalities.
Loading...
Research containing Medable
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Medable in 4 CB Insights research briefs, most recently on Aug 21, 2024.

Aug 21, 2024
The clinical trials tech market map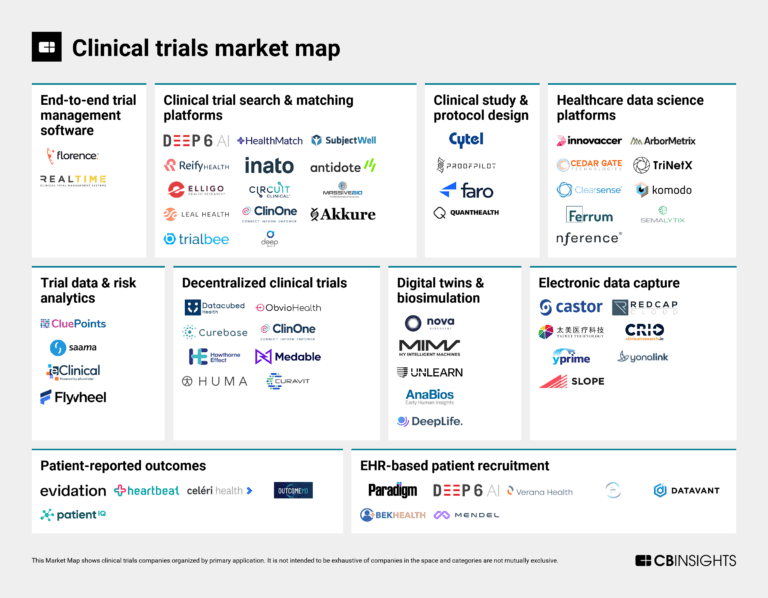
Aug 1, 2023
The clinical trials market mapExpert Collections containing Medable
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Medable is included in 6 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
Regtech
1,453 items
Technology that addresses regulatory challenges and facilitates the delivery of compliance requirements. Regulatory technology helps companies and regulators address challenges ranging from compliance (e.g. AML/KYC) automation and improved risk management.
Conference Exhibitors
5,302 items
Digital Health 50
300 items
The winners of the second annual CB Insights Digital Health 150.
Digital Health
11,305 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Telehealth
3,114 items
Companies developing, offering, or using electronic and telecommunication technologies to facilitate the delivery of health & wellness services from a distance. *Columns updated as regularly as possible; priority given to companies with the most and/or most recent funding.
Medable Patents
Medable has filed 5 patents.
The 3 most popular patent topics include:
- health informatics
- healthcare occupations
- medical terminology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
6/21/2022 | 2/13/2024 | Computer security, Information privacy, Data security, Computer network security, Access control | Grant |
Application Date | 6/21/2022 |
|---|---|
Grant Date | 2/13/2024 |
Title | |
Related Topics | Computer security, Information privacy, Data security, Computer network security, Access control |
Status | Grant |
Latest Medable News
Mar 10, 2025
Medable Inc., a leading provider of clinical development technology, announced that its eConsent and eCOA solutions have been approved for use in two clinical studies in eight countries outside of the European Union by France’s Commission Nationale de l’Informatique et des Libertés (CNIL). This milestone makes Medable uniquely capable of operating as a data processor outside of the CNILMR-001 Reference Methodology requirements for a France-based clinical trial sponsor. The achievement underscores Medable’s commitment to expanding access and providing secure, compliant, and scalable digital solutions for global clinical research. Medable partnered closely with Servier – one of the largest pharmaceutical sponsors in France – over six months to obtain CNIL approval. The team compiled an extensive dossier with platform validation documents, provided demonstrations and videos addressing security questions, and remained flexible in updating workflows and training materials through multiple CNIL revisions. Cross-functional collaboration and persistence across both companies earned the hard-won approval, expanding the use of Medable’s eConsent, eCOA, and other solutions. “This approval is a testament to the strength of our platform and industry collaborations,” said Dr. Michelle Longmire, CEO and Co-Founder of Medable. “By combining regulatory expertise, compliance, innovation, and patient-centricity, we’re reshaping the future of clinical trials.” Medable has deployed its software-as-a-service platform in more than 300 decentralized and digital clinical trials in 70 countries, serving more than one million patients and research participants globally. Customers have achieved impressive results – including 90% eCOA adherence and 50% cost reductions. A Tufts CSDD study also shows that, on average, decentralized trials can achieve net financial benefits from five to 13 times for Phase II and Phase III trials, equating to roughly $10 million ROI and $39 million ROI for an investment on average of $500K in Phase II and $1.5M inPhase III trials, respectively. Recently, the company launched Medable AI and Medable Studio to automatically convert outcomes assessments into fully digital eCOAs in seconds – now accessible on Google Cloud Marketplace. About Medable Medable is on a mission to get effective therapies to people faster. Its digital clinical trials platform enhances speed, scale, and patient access in clinical research, accelerating medicines for thousands of conditions without treatment or cure. Awarded Best Digital Health Solution by the Galien Foundation, Medable’s platform has been deployed in nearly 400 trials in 70 countries and 120 languages, serving more than one million patients globally. Medable is a privately held, venture-backed company headquartered in Palo Alto, California, and was listed for the second year in a row on the Inc. 5000 in 2024. Like what you are reading? Sign up for our free newsletter SIGN ME UP
Medable Frequently Asked Questions (FAQ)
When was Medable founded?
Medable was founded in 2012.
Where is Medable's headquarters?
Medable's headquarters is located at 525 University Avenue, Palo Alto.
What is Medable's latest funding round?
Medable's latest funding round is Series D.
How much did Medable raise?
Medable raised a total of $533.75M.
Who are the investors of Medable?
Investors of Medable include GSR Ventures, Sapphire Ventures, Tiger Global Management, Western Technology Investment, Blackstone and 13 more.
Who are Medable's competitors?
Competitors of Medable include Lindus Health, mpathic, Pattern Health, Castor, ObvioHealth and 7 more.
What products does Medable offer?
Medable's products include Medable Platform and 2 more.
Who are Medable's customers?
Customers of Medable include GSK and Syneos.
Loading...
Compare Medable to Competitors

ObvioHealth provides digital health solutions within the clinical trial industry. The company has a platform and mobile application allows for remote monitoring and participation in clinical trials, focusing on data collection and participant engagement. ObvioHealth's services are relevant to the healthcare sector, especially in trial management. It was founded in 2017 and is based in New York, New York.

THREAD is a company focused on clinical research and electronic clinical outcome assessments (eCOA) within the life sciences sector. It offers a proprietary decentralized research platform and a suite of supporting services designed to enable remote data capture from participants and sites during clinical studies. It was founded in 2005 and is based in Tustin, California.

Curebase provides a range of software tools designed for clinical trial recruitment, consent, and data collection processes. Curebase primarily serves the clinical research industry, including CROs, research sites, and study participants. The company was founded in 2017 and is based in San Francisco, California.

Castor operates within the healthcare sector and provides a clinical trial platform designed for the design, deployment, patient engagement, data collection, and analysis of clinical trials. The platform is adaptable and can meet the requirements of different stakeholders in the clinical research process. It primarily serves the healthcare sector. The company was founded in 2012 and is based in Amsterdam, Netherlands.

Reify Health specializes in optimizing clinical trial operations within the healthcare sector. The company offers cloud-based software solutions designed to accelerate patient enrollment in clinical trials, aiming to streamline the process of bringing new therapies to patients. Reify Health primarily serves the biopharmaceutical industry, research clinics, and healthcare organizations involved in clinical research. Reify Health was formerly known as ZeroSum Health. It was founded in 2012 and is based in Boston, Massachusetts.

Medidata specializes in providing a unified platform for clinical research within the life sciences sector. The company offers a range of products and solutions designed to streamline clinical trials, including data management, clinical operations, and patient engagement technologies. Medidata's platform serves various sectors, including biopharma, medical device companies, and academic research organizations. It was founded in 1999 and is based in New York, New York.
Loading...