
Spectrum Medical
Founded Year
2007Stage
Loan | AliveValuation
$0000About Spectrum Medical
Spectrum Medical specializes in perfusion systems and clinical information solutions for the healthcare sector. The company offers products that support extracorporeal therapies and high acuity healthcare spaces, including medical device connectivity, electronic medical records, and non-invasive diagnostic systems. Spectrum Medical primarily serves the healthcare industry, with a focus on cardiac operating rooms and intensive care units. It was founded in 2007 and is based in Gloucester, United Kingdom.
Loading...
Loading...
Expert Collections containing Spectrum Medical
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Spectrum Medical is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
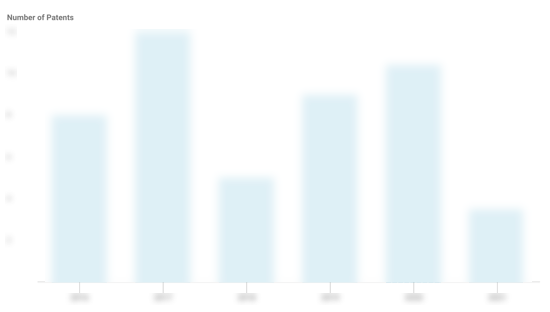
Spectrum Medical Patents
Spectrum Medical has filed 28 patents.
The 3 most popular patent topics include:
- fluid dynamics
- medical equipment
- respiratory physiology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
7/17/2018 | 3/4/2025 | Cardiac surgery, Cooling technology, Heat exchangers, Medical equipment, Energy recovery | Grant |
Application Date | 7/17/2018 |
|---|---|
Grant Date | 3/4/2025 |
Title | |
Related Topics | Cardiac surgery, Cooling technology, Heat exchangers, Medical equipment, Energy recovery |
Status | Grant |
Latest Spectrum Medical News
Sep 19, 2024
News Provided By Share This Article Cardiopulmonary Bypass Accessory Equipment Global Market Report 2024 – Market Size, Trends, And Global Forecast 2024-2033 The Business Research Company's Cardiopulmonary Bypass Accessory Equipment Global Market Report 2024 – Market Size, Trends, And Global Forecast 2024-2033 You Can Now Pre Order Your Report To Get A Swift Deliver With All Your Needs” — The Business Research company LONDON, GREATER LONDON, UNITED KINGDOM, September 19, 2024 / EINPresswire.com / -- The cardiopulmonary bypass accessory equipment market has experienced robust growth in recent years, expanding from $14.63 billion in 2023 to $15.6 billion in 2024 at a compound annual growth rate (CAGR) of 6.6%. The growth in the historic period can be attributed to increasing awareness of cardiovascular disease, growing specialized cardiothoracic surgery programs, increasing number of organ transplant survivors, rising cases of cardiac illnesses, and a growing aging population. What Is The Estimated Market Size Of The Global Cardiopulmonary Bypass Accessory Equipment Market And Its Annual Growth Rate? The cardiopulmonary bypass accessory equipment market is projected to continue its strong growth, reaching $20.28 billion in 2028 at a compound annual growth rate (CAGR) of 6.8%. The growth in the forecast period can be attributed to the rising prevalence of cardiovascular disease, growing prevalence of acute respiratory failure, expanding and modernizing healthcare facilities, increasing funding and investments, and increasing medical tourism. Explore Comprehensive Insights Into The Global Cardiopulmonary Bypass Accessory Equipment Market With A Detailed Sample Report: Growth Driver Of The Cardiopulmonary Bypass Accessory Equipment Market The growing prevalence of acute respiratory failure is expected to propel the growth of the cardiopulmonary bypass accessory equipment market. Acute respiratory failure occurs when the respiratory system fails in one or both of its gas exchange activities, such as oxygenation and carbon dioxide removal. Acute respiratory failure is rising due to several factors, including the aging population, infectious diseases, and medical procedures. Cardiopulmonary bypass equipment responds to the demand for advanced respiratory support by providing comprehensive oxygenation and ventilation management in ICUs and hospitals, effectively maintaining complex situations of respiratory distress. Order Your Report Now For A Swift Delivery: Key players in the market include Abbott, Medtronic, Fresenius Medical Care, Terumo Corporation, Edwards Lifesciences Corporation, B. Braun Medical, NIPRO, Getinge AB, ABIOMED, LivaNova Inc., MicroPort Scientific Corporation, Nonin, Spectrum Medical, Braile Biomédica, Mercury Medical, EUROSETS, Chalice Medical Ltd., Surge Cardiovascular, Andocor, Senko Medical Instrument Mfg. Co. Ltd. Major companies operating in the cardiopulmonary bypass accessory equipment market are developing advanced perfusion systems to ensure safe and effective cardiopulmonary bypass procedures through advanced features and better clinical workflows. The perfusion system comprises a next-generation heart-lung machine (HLM) and precise sensing technology for data-driven decision-making for safe cardiopulmonary bypass (CPB) management. How Is The Global Cardiopulmonary Bypass Accessory Equipment Market Segmented? 1) By Product Type: Single Roller Pump, Double Roller Pump 2) By Operation: Manually Operated, Electrically Operated, Battery Operated 3) By Distribution Channel: Direct Tender, Third-Party Distributor, Retail Sales 4) By Application: Lung Transplant Operation, Acute Respiratory Failure Treatment, Cardiac Surgery, Other Applications 5) By End-Use: Hospitals, Ambulatory Care Centers, Surgical Centers, Other End-Uses Geographical Insights: North America Leading The Cardiopulmonary Bypass Accessory Equipment Market North America was the largest region in the cardiopulmonary bypass accessory equipment market in 2023. The regions covered in the report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. Cardiopulmonary Bypass Accessory Equipment Market Definition Cardiopulmonary bypass (CPB) accessory equipment are devices and instruments used during cardiac surgery with CPB to keep blood circulation and oxygenation outside the body after the heart stops. These accessories regulate blood temperature and filter out microemboli, protecting the patient's systemic circulation. Cardiopulmonary Bypass Accessory Equipment Global Market Report 2024 from TBRC covers the following information: •Market size data for the forecast period: Historical and Future •Macroeconomic factors affecting the market in the short and long run •Analysis of the macro and micro economic factors that have affected the market in the past five years •Market analysis by region: Asia-Pacific, China, Western Europe, Eastern Europe, North America, USA, South America, Middle East and Africa. •Market analysis by countries: Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA. An overview of the global cardiopulmonary bypass accessory equipment market report covering trends, opportunities, strategies, and more The Cardiopulmonary Bypass Accessory Equipment Global Market Report 2024 by The Business Research Company is the most comprehensive report that provides insights on cardiopulmonary bypass accessory equipment market size, cardiopulmonary bypass accessory equipment market drivers and trends, cardiopulmonary bypass accessory equipment competitors' revenues, cardiopulmonary bypass accessory equipment market growth across geographies. This report helps you gain in-depth insights into opportunities and strategies. Companies can leverage the data in the report and tap into segments with the highest growth potential. Browse Through More Similar Reports By The Business Research Company: Cardiovascular Surgery Devices And Equipment Global Market Report 2024
Spectrum Medical Frequently Asked Questions (FAQ)
When was Spectrum Medical founded?
Spectrum Medical was founded in 2007.
Where is Spectrum Medical's headquarters?
Spectrum Medical's headquarters is located at Cheltenham Road East, Gloucester.
What is Spectrum Medical's latest funding round?
Spectrum Medical's latest funding round is Loan.
Who are the investors of Spectrum Medical?
Investors of Spectrum Medical include HSBC UK, CVC Capital Partners and USC/Columbia Technology Incubator.
Who are Spectrum Medical's competitors?
Competitors of Spectrum Medical include HydroCision, Clearflow, Mitralign, Dextera Surgical, ReShape Medical and 7 more.
Loading...
Compare Spectrum Medical to Competitors

USGI Medical develops endoluminal technologies for the healthcare sector and focuses on surgical solutions. The company offers products that enable incision-free procedures, including endoluminal suturing systems for tissue approximation and Snowshoe Suture Anchors for full-thickness plications. USGI Medical's technologies aim to provide solutions in gastroenterology. It was founded in 2001 and is based in San Clemente, California.

HydroCision focuses on minimally invasive surgery technology in the orthopedic and spine sectors. The company offers products that use fluidjet technology to perform surgical procedures with a nonthermal saline stream, allowing for the cutting and aspiration of tissue without external suction. Its technology removes diseased tissue and aims to preserve surrounding healthy tissue, relevant to sports medicine and spine applications. It was founded in 1994 and is based in North Billerica, Massachusetts.
NeoChord focuses on beating heart mitral valve repair within the healthcare sector. The company offers the neochord artificial chordae delivery system, an echo-guided treatment for mitral valve regurgitation. It provides consultation services and resources for patients and healthcare professionals. NeoChord was formerly known as mValve. It was founded in 2007 and is based in Osseo, Minnesota.
Clearflow engages in improving patient outcomes in the medical sector, specifically in cardiac surgery. It offers medical technologies that help prevent complications due to retained blood after heart and lung surgeries, such as blood clot obstructions in chest tubes. It enables healthcare professionals to manage postoperative bleeding. It was formerly known as Clear Catheter Systems. The company was founded in 2007 and is based in Irvine, California.
Arbor Surgical Technologies, Inc. with facilities located in Irvine and Portola Valley, CA (USA), is a privately held cardiovascular medical device company focused on the heart valve replacement market. Founded in 2002 by Dr. Thomas J. Fogarty and noted heart valve designer, Ernie Lane, Arbor is developing multiple technologies that have the potential to deliver significant clinical benefit to patients worldwide. Arbor intends to develop and commercialize its core technologies in varying combinations, giving the company the opportunity to create a broad portfolio of product offerings. Currently in clinical trials, Arbor is developing a tissue heart valve family of products designed for improved performance over existing valves and compatible implantation tools that simplify and speed valve replacement surgery. Arbor Surgical Technologies mission is to simplify cardiac valve surgery through the innovative development of a less invasive, unique implantation system and valves with superior performance and durability. Partnering with physicians, we will improve patient recovery by less invasive techniques and reduced surgery time. Ethical behavior with employees, surgeons, patients and suppliers is the keystone to our success.
Axis Surgical Technologies, Inc. aims to develop, manufacture, and market devices that improve quality and efficiency in spinal surgery, especially for minimally invasive surgical techniques. Based in Mountain View, California, Axis offers an integration of visualization and articulation capabilities and provides spinal surgery equipment. Axis' FDA Grant In March 2010, Axis announced that it received 510(k) clearance from the Food and Drug Administration to market its C-MOR„ Visualization Device to use in diagnostic and operative arthroscopic and endoscopic procedures. This portable tool provides visualization and illumination of an interior cavity through either a natural or surgical opening. The ergonomic lightweight device also offers practitioners the convenience of endoscopic visualization and efficient one-handed operating ability; this device can be employed in hospital outpatient departments, ambulatory surgery centers, and office surgery suites.
Loading...
